![Stoichiometry - Lecture notes 19 - To p of Page g NaOH = 500 mL soln [NaOH] = moles/L sola 2 - Studocu Stoichiometry - Lecture notes 19 - To p of Page g NaOH = 500 mL soln [NaOH] = moles/L sola 2 - Studocu](https://d3tvd1u91rr79.cloudfront.net/1b2a15468c5e93da0584fa127feccda5/html/bg1.png?Policy=eyJTdGF0ZW1lbnQiOlt7IlJlc291cmNlIjoiaHR0cHM6Ly9kM3R2ZDF1OTFycjc5LmNsb3VkZnJvbnQubmV0LzFiMmExNTQ2OGM1ZTkzZGEwNTg0ZmExMjdmZWNjZGE1L2h0bWwvKiIsIkNvbmRpdGlvbiI6eyJEYXRlTGVzc1RoYW4iOnsiQVdTOkVwb2NoVGltZSI6MTY4NDkxMDI2OH19fV19&Signature=R~20HVX4-ruHonr~HeB6BcbdBhEDIaJJTWjxQN8LvHTzIT4tdCXo7BYj1YgjBCw6CbR~Bi-rwtdAcyO018cGWsSZzFD1ABlxtcBf9PT52uHKnLJXh4-MLrE2RpH-5l0sVpE7DAVp-UGQmcyTtW14N9X4LJZBrYqYZd-zEEJurFM~tvzD7yFkHL5E2sKWYNNWig0yLQKbkh-3stYYXjlS9WYKYN6q8x-VGryiBrCMyq~Ujrfro0K4XJe0ikLrwMpteS8nlbTvZuuk7VvUGbogdHhSz-jTSZqa6XWLygJLlgGzBIVjegUKmbTGZDN9TBFrrv5cuMFEyM60P5AtqZxbgw__&Key-Pair-Id=APKAJ535ZH3ZAIIOADHQ)
Stoichiometry - Lecture notes 19 - To p of Page g NaOH = 500 mL soln [NaOH] = moles/L sola 2 - Studocu

Effects of sodium hydroxide's concentration and time on the yields of... | Download Scientific Diagram

Stock of labile (Resin-P, NaHCO3–Pi,o), moderately labile (NaOH–Pi,o),... | Download Scientific Diagram
![C/P Section Bank #14] I understand why we use NaOH in the first step. However, I don't understand why choosing to use diethyl ether over HCl. Can't the HCl react with the C/P Section Bank #14] I understand why we use NaOH in the first step. However, I don't understand why choosing to use diethyl ether over HCl. Can't the HCl react with the](https://i.redd.it/lr1b4v0aahk11.jpg)
C/P Section Bank #14] I understand why we use NaOH in the first step. However, I don't understand why choosing to use diethyl ether over HCl. Can't the HCl react with the

Cell Wash Solution I /Naoh-D 2L Roche Reagent Modular P/D Cobas C702 - China Cell Wash Solution I /Naoh-D and Roche Reagent
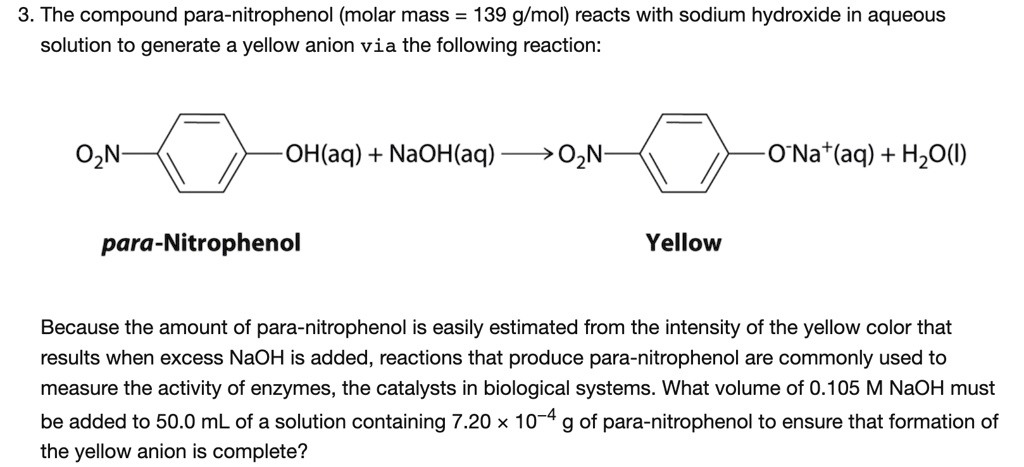
SOLVED: 3. The compound para-nitrophenol (molar mass = 139 g/mol) reacts with sodium hydroxide in aqueous solution to generate a yellow anion via the following reaction: OzN OH(aq) + NaOH(aq) O2N 0

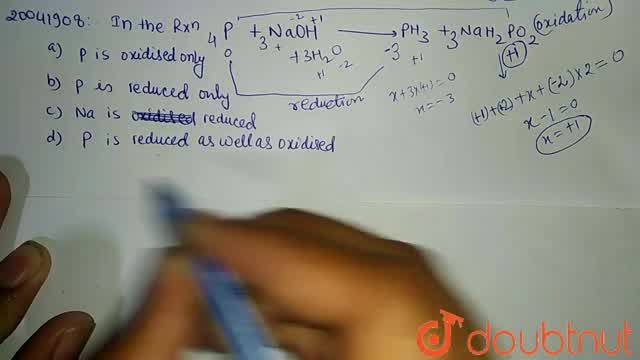
White phosphorus on reaction with concentrated NaOH solution in an inert atmosphere of CO2 gives phosphine and compound (X) . (X) on acidification with HCl gives compound (Y) . The basicity of

Historical changes of sedimentary P-binding forms and their ecological driving mechanism in a typical “grass-algae” eutrophic lake – Freshwater Ecology

Predict the product of the reaction of p-methylbenzoic acid with the stated reagent. NaOH, then CH3I | Homework.Study.com

i) Out of and which one is more reactive towards S N 1 and why? (ii) Write the product formed when p-nitrochlorobenzene is heated with aqueos NaOH at 443 K followed by
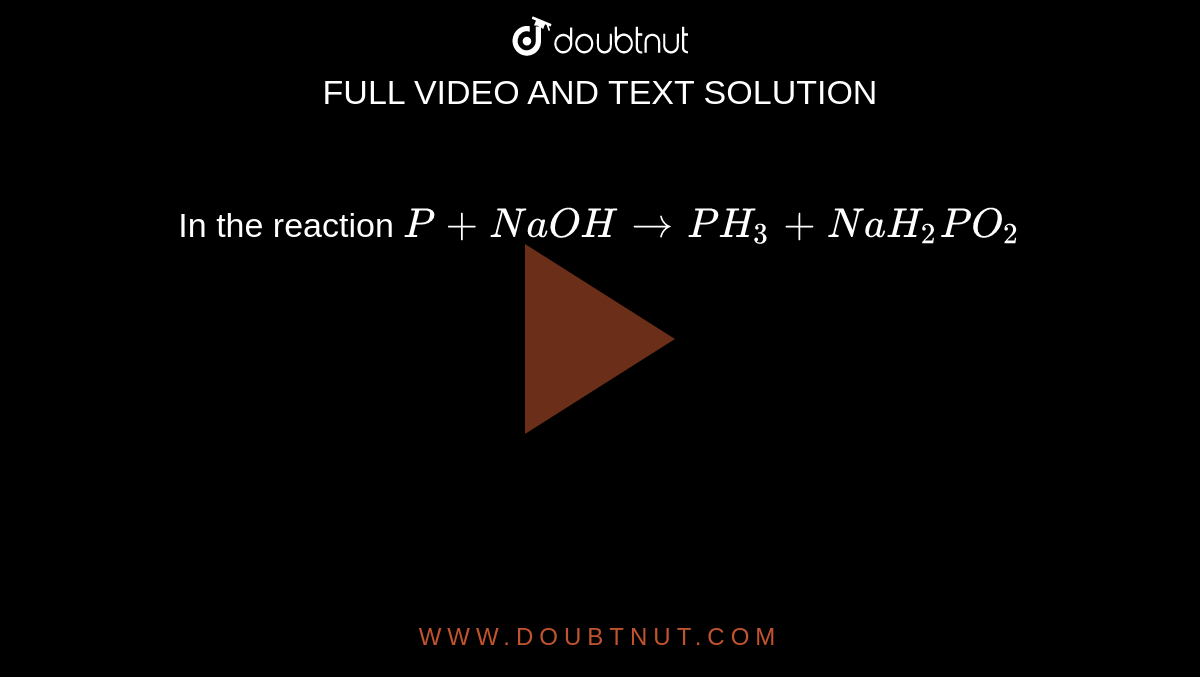
How should I balance this equation P + NaOH + H2O---------> PH3 + NaH2PO2 by ion electron method? - Quora

Write an equation for the reaction of p-bromobenzaldeyde with HCN (NaOH as the catalyst). | Homework.Study.com

Water | Free Full-Text | Phosphorus Forms and Associated Properties along an Urban–Rural Gradient in Southern China




![ANSWERED] Consider the following three solutions of... - Physical Chemistry ANSWERED] Consider the following three solutions of... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/59737718-1659709481.9039986.jpeg)